WELLBUTRIN XL Savings & Access Program
We are here to help patients access and afford WELLBUTRIN XL. The WELLBUTRIN XL Savings & Access Program consists of:
Prior authorization (PA) supportPA CONSIDERATIONS >> Request Live Reimbursement Support >>

Resources to help healthcare professional teams manage the PA process
PA CONSIDERATIONS FOR WELLBUTRIN XL >>
Copay savingsENROLLMENT >>CARD ACTIVATION >>

Most eligible* commercially insured patients may pay as low as $5 for a 30-day supply
*This offer is not valid for patients covered by Medicare, Medicaid or any other federal or state funded healthcare program or where prohibited by law. Please see full eligibility criteria above.
Free delivery of WELLBUTRIN XL DOWNLOAD FORM >>
PhilRx program highlights
- Deliver medications directly to the patient through an independent pharmacy network
- Apply a monthly copay as low as $5 for eligible* commercially insured patients
- Schedule refills
- Troubleshoot insurance
Get started with PhilRx
1. Healthcare professionals should send a patient’s WELLBUTRIN XL prescription to PhilRx
2. PhilRx will process it and text the patient for insurance and delivery information
*This offer is not valid for patients covered by Medicare, Medicaid or any other federal or state funded healthcare program or where prohibited by law. Please see full eligibility criteria above.
Assistance with insurance coverage REGISTER NOW >>

WELLBUTRIN XL support from CoverMyMeds® allows healthcare professionals to:
- Manage quick and efficient prior authorizations
- Receive fast approvals
- Automatically renew previous prior authorizations
Reasons to Prescribe a Brand Name Experience
The generic versions of WELLBUTRIN XL may look considerably different from the original branded product
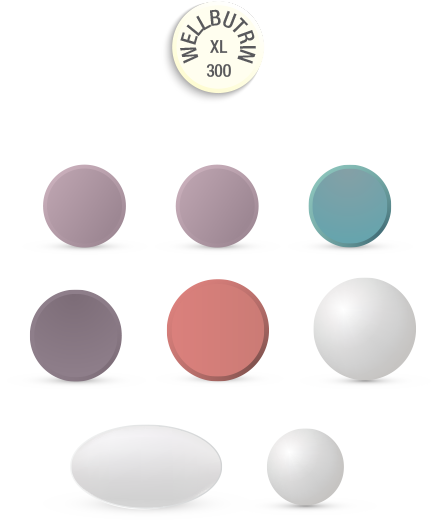
Graphic representation of WELLBUTRIN XL and generic versions of WELLBUTRIN XL. Not actual size.
- Changes in sizes, shapes, colors, tastes, smells, and packaging of generic medications compared with branded drug can potentially impact treatment adherence3,4
- Patients and healthcare professionals can find it difficult to correctly identify medications, which can lead to errors3,4
- In a study in patients with MDD, researchers found that treatment adherence and persistence were higher in patients treated with 3 branded antidepressants (serotonin reuptake inhibitors) vs those receiving the generic alternatives5,6
Write/Select DAW1, Brand Medically Necessary on your WELLBUTRIN XL scripts
to help ensure patients get the brand name product and potentially avoid adherence issues.3,4
*Disclaimer: This code is for informational purposes only. It represents no statement, promise, or guarantee by Bausch Health Companies Inc. concerning coverage and/or levels of reimbursement, payment, or charge and is not intended to increase or maximize reimbursement by any payer. It is the responsibility of the healthcare provider to determine the appropriate code(s) for service provided to his or her patient.
Free Samples
Get your patients started on WELLBUTRIN XL in the office with free samples.
References:
- WELLBUTRIN XL (bupropion hydrochloride extended-release) Prescribing Information. Bausch Health Companies Inc.
- Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2003/21515ltr.pdf. Accessed May 10, 2023.
- Straka RJ, Keohane DJ, Liu LZ. Potential clinical and economic impact of switching branded medications to generics. Am J Ther. 2017;24(3):e278-e289.
- Peters JR. From our perspective: the importance of the physical characteristics of generic drugs. U.S. Food & Drug Administration. https://www.fda.gov/drugs/newsevents/ucm471446.htm. Accessed May 10, 2023.
- Italiano D, Spina E. Generic antidepressant drugs: a reappraisal. J Psychopathology. 2013;19(3):I-V.
- Liu X, Chen Y, Faries DE. Adherence and persistence with branded antidepressants and generic SSRIs among managed care patients with major depressive disorder. Clinicoecon Outcomes Res. 2011;3:63-72.




